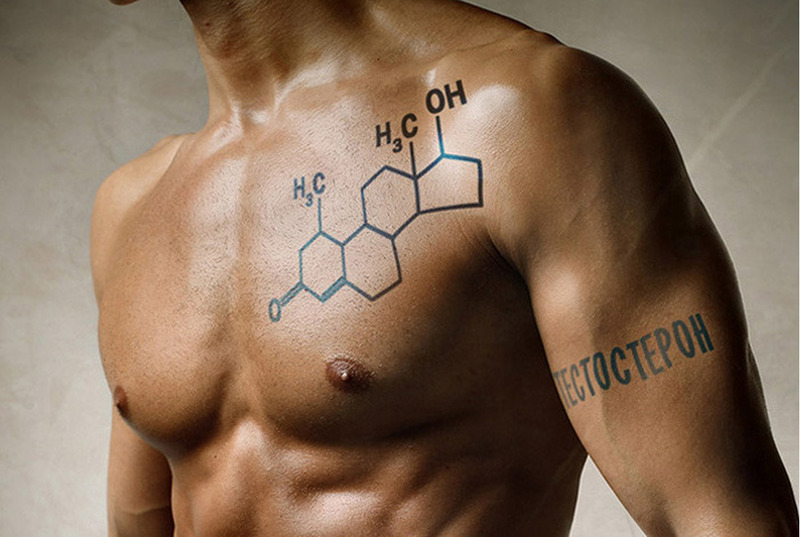
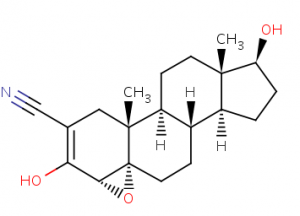
 Trilostane [ (4α, 5α, 17β) -3,17-dihydroxy-4,5-epoxyhydrost-2-en-2-carbonitrile ] is an inhibitor adrenocortical steroidogenesis. The molecule inhibits the enzyme progesterone reductase, which is necessary for the production of glycorticoids and mineralocorticoids, competitively and reversibly blocking the conversion of D5-3b-hydroxysteroids, for example, pregnenolone, which is biologically inactive, into D4-3-ketosteroids, as well as, For example, biologically active progesterone, both in the adrenal cortex and in other tissues. Trilostane appears to be more effective in people with hypercortisolism than in people with normal adrenocortical activity. The drug will also lead to inhibition of 3-beta-hydroxysteroid dehydrogenase.
Trilostane [ (4α, 5α, 17β) -3,17-dihydroxy-4,5-epoxyhydrost-2-en-2-carbonitrile ] is an inhibitor adrenocortical steroidogenesis. The molecule inhibits the enzyme progesterone reductase, which is necessary for the production of glycorticoids and mineralocorticoids, competitively and reversibly blocking the conversion of D5-3b-hydroxysteroids, for example, pregnenolone, which is biologically inactive, into D4-3-ketosteroids, as well as, For example, biologically active progesterone, both in the adrenal cortex and in other tissues. Trilostane appears to be more effective in people with hypercortisolism than in people with normal adrenocortical activity. The drug will also lead to inhibition of 3-beta-hydroxysteroid dehydrogenase.
In a patient with Cushing’s syndrome, trilostane usually reduces the secretion, plasma concentration and urinary excretion of cortisol and inhibits the adrenocortical response to corticotropin stimulation (ACTH). During treatment with the drug over long periods of time, an increase, due to a negative feedback mechanism, the concentration of ACTH in plasma may occur, followed by stimulation of adrenal steroidogenesis. This mechanism in some subjects can lead to the cancellation of the inhibition of cortisol synthesis.
After oral administration, trilostane is usually rapidly absorbed from the gastrointestinal tract. It is also known that there are wide interindividual differences in the rate and extent of absorption.
In healthy subjects on an empty stomach, approximately 30-60 minutes after oral administration of a single 120 mg dose, the drug can be administered in plasma. The maximum plasma concentrations of trilostane and 17-ketotriostane are 0.4-1 and 1.2-2.5 μg / ml, respectively. The drug is widely distributed in most body tissues, especially in the adrenal glands, liver, lungs and kidneys. Trilostane is metabolized by the liver. 5 major metabolites have been found in animals after hydroxylation and glucuronation reactions.
The main metabolite is 17-ketotrylostane, which appears to be twice as strong as trilostane in inhibiting progesterone reductase. In monkeys, trilostane and its metabolites are excreted mainly in the urine, in rats – in the feces. Oral LD50 values in rats and mice are> 16 g / kg.
Trilostane is used to treat Cushing’s syndrome and hyperaldosteronism.
It is also used to treat advanced breast cancer in postmenopausal women.
The usual therapeutic dose of trilostane is 60 mg 4 times daily for at least 3 days; it is then adjusted based on the patient’s response in the range of 120 to 480 mg / day. Daily doses of 960 mg / day have also been introduced.
Many bodybuilders use trilostane for its ability to inhibit cortisol synthesis; which can come in handy, for example, when exiting long AAS cycles to avoid excessive muscle loss and gain consolidation, or when severe and prolonged calorie restriction occurs. In fact, this molecule, or Metyrapone, is increasingly being used in place of Cytadren (Aminoglutetimide), often used by athletes in the past.
Cortisol is an important element in the equation that causes genetic restrictions on muscle growth. Many insiders and athletes understand that:
- less cortisol = less muscle catabolism (breakdown);
- less estrogen = less fat storage and less gynecomastia and therefore a leaner body;
- less aldosterone = less water retention and a more toned appearance.
But less conversion of pregnenolone to active compounds = less endogenous testosterone.
Many consider these highly beneficial effects to be a lack of natural testosterone production through the use of AAS, and it has been recognized that the lack of competing hormones had a profound synergistic effect on auto-hormones. -Administered. Therefore, it should be remembered that trilostane, like aminoglutethimide, does not include a reduction solely for cortisol: in fact, its inhibitory powers also proportionally affect the endogenous biosynthesis of androgens, estrogens and aldosterone. For this reason, athletes take Proviron (Mesterolone) while taking trilostane to maintain high circulating androgen levels.
Doses for athletes are usually in the range of 120-240 mg per day. it is divided into several doses of 60 mg, as a rule, with two days and two days without a protocol for a maximum of four weeks q. This way, you can make the most of the molecule’s potential without suffering harmful negative feedback loops and reducing the frequency of other potential side effects. The use of Trilostane did not last more than 4-6 weeks: at this point, the body would react by increasing the production of ACTH, creating a whole new series of catabolic effects.
The Trilostano protocol, where the goal is to suppress cortisol and estrogen, could be (as a pure example) the following:
Days
- Trilostane 120 mg, Proviron 50/150 mg
- Trilostane 120 mg, Proviron 50/150 mg
- Nolvadex 30 mg, Proviron 50/150 mg
- Nolvadex 30 mg, Proviron 50/150 mg
- Trilostane 120 mg, Proviron 50/150 mg
- Trilostane 120 mg, Proviron 50/150 mg
- Nolvadex 30 mg, Proviron 50/150 mg
- Nolvadex 30 mg, Proviron 50/150 mg
- Trilostane 120 mg, Proviron 50/150 mg
- Trilostane 120 mg, Proviron 50/150 mg
- Nolvadex 30 mg, Proviron 50/150 mg
- Nolvadex 30 mg, Proviron 50/150 mg
- Trilostane 180 mg, Proviron 50/150 mg
- Trilostane 180 mg, Proviron 50/150 mg
- Nolvadex 30 mg, Proviron 50/150 mg
- Nolvadex 30 mg, Proviron 50/150 mg
- Trilostane 180 mg, Proviron 50/150 mg
- Trilostane 180 mg, Proviron 50/150 mg
- Nolvadex 30 mg, Proviron 50/150 mg
- Nolvadex 30 mg, Proviron 50/150 mg
- Trilostane 240 mg, Proviron 50/150 mg
- Trilostane 240 mg, Proviron 50/150 mg
- Nolvadex 30 mg, Proviron 50/150 mg
- Nolvadex 30 mg, Proviron 50/150 mg
- Trilostane 240 mg, Proviron 50/150 mg
- Trilostane 240 mg, Proviron 50/150 mg
- Nolvadex 30 mg, Proviron 50/150 mg
- Nolvadex 30 mg, Proviron 50/150 mg
The following side effects have occurred after taking high doses of trilostane:
- headache
- asthenia
- exhaustion
- feeling empty head
- malaise
- confusion
- flush
- nausea
- vomiting
- diarrhea
- rhinorrhea and palatal edema
- liver problems.
Skin peeling, rash, itching, and erythema have sometimes been reported. Rarely, arthralgia, cramps or muscle pains, palpitations, turgor and congestion in the nasal mucosa, lacrimation, fever, fainting and increased salivation may occur.
As mentioned above, trilostane can inhibit gonadal function. A woman who received 240 mg of the drug 4 times a day developed an Addison crisis. The drug also severely reduces the body’s ability to respond to inflammatory responses. This means that it can prevent the body from stopping bleeding and fighting disease. It can also make a bodybuilder a victim of Cushing’s syndrome. An overdose of trilostane occurs with hypotension, even with severe, hyperkalemia and adrenal insufficiency. For treatment, the stomach must be emptied with vomiting or gastric lavage and corticosteroid replacement therapy. Serum potassium and blood pressure must be closely monitored.
Trilostane is structurally related to testosterone and may interfere with some serum testosterone determination methods. The drug can also give falsely high values of 11-hydroxycorticosteroids in urine and / or plasma.