 Metabolic damage is a question that has always wondered anyone who decides to approach a diet for weight loss if, on the one hand, a drastic reduction in calorie intake leads to rapid and concrete weight loss, on the other hand, will have implications for various aspects, such as the breakdown of macronutrients and partly into subtle hormonal aspects associated with the management of the macronutrients themselves.
Metabolic damage is a question that has always wondered anyone who decides to approach a diet for weight loss if, on the one hand, a drastic reduction in calorie intake leads to rapid and concrete weight loss, on the other hand, will have implications for various aspects, such as the breakdown of macronutrients and partly into subtle hormonal aspects associated with the management of the macronutrients themselves.
Before starting to tackle the problem specifically, it is useful to remember What are the main functions of each macronutrient that they cover. If you feel sufficiently prepared on this subject, you can skip this article, in which I admit it, I may have gone too far, but it is not easy to summarize something as broad as what we will be dealing with now. always remember, even when you lose your urge to go all the way, what you read is an EXTREME summary of what one could say about these topics.
Carbohydrates
 We are used to thinking of this macronutrient as the main source of energy, and this is certainly true, but this is certainly an oversimplification, and since we are trying to deepen it, we leave excessive simplifications (and I would add introductory delusion) at other times, Carbohydrates constitute a group of fundamental organic substances of living matter. They are widespread in nature, especially in the plant kingdom, where they play mainly structural roles (such as cellulose) or as a reserve material (starch). In animal organisms, on the other hand, they are present both in free form in the form of carbohydrates and also associated with protides (for example, we often find molecules such as glycosaminoglycans, associated with proteins to form proteoglycans, which play a mechanical auxiliary role together with molecules such as collagen); now it will be legal to ask all this to say what? Just to make it clear that the roles of each macronutrient are not limited to what we usually think they do, just think that deoxy-D-ribose, together with a nitrogenous base and a phosphate group, forms a nucleotide, that is, the fundamental unit of DNA. Since we are doing a little “lively” biochemistry, it is necessary to indicate the chemical composition of these molecules, which are composed of C (carbon), H (hydrogen) and O (oxygen), even if some of them have other elements as well, such as N (nitrogen ) in amino sugars and S (sulfur) in thiosugars.
We are used to thinking of this macronutrient as the main source of energy, and this is certainly true, but this is certainly an oversimplification, and since we are trying to deepen it, we leave excessive simplifications (and I would add introductory delusion) at other times, Carbohydrates constitute a group of fundamental organic substances of living matter. They are widespread in nature, especially in the plant kingdom, where they play mainly structural roles (such as cellulose) or as a reserve material (starch). In animal organisms, on the other hand, they are present both in free form in the form of carbohydrates and also associated with protides (for example, we often find molecules such as glycosaminoglycans, associated with proteins to form proteoglycans, which play a mechanical auxiliary role together with molecules such as collagen); now it will be legal to ask all this to say what? Just to make it clear that the roles of each macronutrient are not limited to what we usually think they do, just think that deoxy-D-ribose, together with a nitrogenous base and a phosphate group, forms a nucleotide, that is, the fundamental unit of DNA. Since we are doing a little “lively” biochemistry, it is necessary to indicate the chemical composition of these molecules, which are composed of C (carbon), H (hydrogen) and O (oxygen), even if some of them have other elements as well, such as N (nitrogen ) in amino sugars and S (sulfur) in thiosugars.
Now let’s move on to a simple classification of sugars (or carbohydrates) with examples that you will all know at least from rumors.
Monosaccharides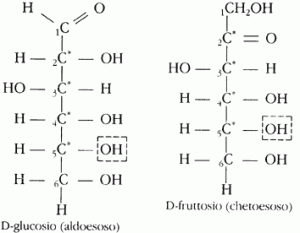 They are the simplest structural units of carbohydrates (my teacher made me learn almost by heart the definition of “are derivatives of aldehydes and ketones of polyvalent alcohols”, but this is not the place to go deeper … just need to know that each monosaccharide can be aldose or ketosis depending on whether it comes from an aldehyde or a ketone), in any case, monosaccharides are molecules that we then actually use for metabolic purposes; they are the end result of the digestion of more complex molecules. The most famous example of a monosaccharide is glucose (which in the bodybuilding world has always been called dextrose, that is, the dextrogenic enantiomer of glucose).
They are the simplest structural units of carbohydrates (my teacher made me learn almost by heart the definition of “are derivatives of aldehydes and ketones of polyvalent alcohols”, but this is not the place to go deeper … just need to know that each monosaccharide can be aldose or ketosis depending on whether it comes from an aldehyde or a ketone), in any case, monosaccharides are molecules that we then actually use for metabolic purposes; they are the end result of the digestion of more complex molecules. The most famous example of a monosaccharide is glucose (which in the bodybuilding world has always been called dextrose, that is, the dextrogenic enantiomer of glucose).
Oligosaccharides (disaccharides, trisaccharides, etc.)
The simplest oligosaccharide is a disaccharide, and there is no better definition than to say that it is a condensation (with the relative elimination of a water molecule) from 2 monoses (or monosaccharides). We have many famous disaccharides, everyone knows, for example, sucrose (common culinary sugar), made up of glucose and fructose, or lactose, a nightmare for many intolerable substances made up of glucose and galactose.
Polysaccharides
Polysaccharides are complex carbohydrates with high molecular weight, they are made up of long chains (which can also be branched) of monosaccharides linked together through glycosidic bonds. Basically, these molecules play 2 fundamental roles:
- Energy storage : they are a material that can be easily used for energy purposes by hydrolysis of individual monosacrides; the most famous and much loved example is glycogen (liver or muscle), which is made up of very long glucose chains.
- Support: Due to their relatively rigid structure, they are functional supports (especially for plant organisms). The most famous example this time is cellulose.
PROTEIN
 If I told you that protein comes from the Greek “protos”, which means, firstly, because they are the main substances of living matter, it would already be galvanized enough to make% of proteins “splash out” in your a rapidly growing diet, so I’ll just say that protein is very important. First of all, they (and even before they become part of our beloved biceps) are fundamental components of many cellular organelles, such as the nucleus and the cytoplasm itself. Protids are widespread both in the animal and plant world, since “life” simply does not exist in their absence; in our body, they make up about 50% of organic material and about 15-17% of the total body weight. Let’s talk about the role of these molecules now, and here you can really indulge yourself.
If I told you that protein comes from the Greek “protos”, which means, firstly, because they are the main substances of living matter, it would already be galvanized enough to make% of proteins “splash out” in your a rapidly growing diet, so I’ll just say that protein is very important. First of all, they (and even before they become part of our beloved biceps) are fundamental components of many cellular organelles, such as the nucleus and the cytoplasm itself. Protids are widespread both in the animal and plant world, since “life” simply does not exist in their absence; in our body, they make up about 50% of organic material and about 15-17% of the total body weight. Let’s talk about the role of these molecules now, and here you can really indulge yourself.
Enzymatic function
Obviously, we are not aware of this, but our body is an almost continuous site of chemical reactions, useful for creating optimal conditions for life. However, most of these reactions would be extremely slow if it were not for the presence of real biological catalysts such as enzymes. In order for the “layman” to understand what we are talking about, enzymes are all those molecules that end in “asi” (for example, hydrolase, synthase, kianci, phosphatase, polymerase, dehydrogenase, aromatase, etc.), about which it follows remember. that these proteins are very specific; but for what? they are very specific to the so-called “ligand” or substrate; it has been used in the past to explain the analogy with the “key lock” theory, which would undoubtedly be useful for introducing the idea of ”enzymatic specificity”, but not very useful for explaining the decrease in parameters such as the activation energy of catalyzed reactions and as a consequence, we started think about the progressive adaptation of the enzyme to the substrate when it comes into contact with it.
Transport function
Proteins are very important for the transport of molecules in our body, among the most famous we have hemoglobin and myoglobin for oxygen transport, the first in the blood and the second in the muscle. Within the framework of cellular respiration metabolism, we find cytochromes used for the transport of electrons (or, better, reducing equivalents) during oxidative phosphorylation, or always in the blood, we find transport lipoteins, the most famous of which are HDL and LDL for cholesterol, but we also there is VLDL (very low density lipoprotein) and chylomicrons for exogenous lipids.
Structural Function
This is probably their primary role or, in any case, the one for which they are best known, as proteins make up real supporting tissues like collagen and provide stability and protection to organs and other tissues.
Hormonal function
Many hormones, such as glucagon, insulin, gh, are real proteins that transmit messages from the endocrine organ to the target organ; however, once they reach the target organ, these hormones cannot penetrate the cell membrane (as steroid hormones do) due to their large size, therefore, they must bind to specific receptors expressed by the target cell and perform their functions through the use of so-called secondary messengers arising from cascade reactions originating from the hormone-receptor connection.
Protective and immune function
Proteins are also very important for our immune system, because its main “defense weapon” against pathogens, antibodies, are proteins produced by cells. specifications such as type B lymphocytes.
Regulatory function
Many proteins are involved in the control of gene expression at the level of RNA transcription.
Homeostatic function
Several proteins are involved in maintaining the stability of body temperature or blood pH.
Energy Function
Probably the most dangerous function for anyone looking to increase muscle mass, yes, proteins with their 4 kcal per gram are able to satisfy our body’s energy needs, this happens mainly when there are no faster sources, such as carbohydrates. In addition, in eggs and seeds, we have the only cases where proteins serve as an energy store.
These are probably not the functions these unusual molecules are capable of performing, but I think they provide a good idea of why they are considered fundamental to life.
All protids mainly consist of four elements: C (carbon), H (hydrogen), O (oxygen), N (nitrogen); from the breakdown of proteins, a mixture formed from amino acids is obtained, even if using processes such as enzymatic hydrolysis, it has been observed that sometimes small amounts of starches and carbohydrates in general may be present; but without overcomplicating life, we can view amino acids as the building blocks of complex protein structures. Biochemically speaking, amino acids are organic acids in the structural formula of which there is at least one amino group (-NH2) linked to one or more carbon atoms.
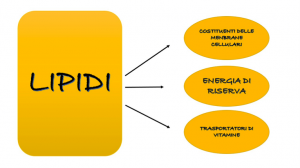
LIPID
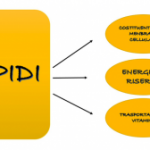 This name indicates a substance with a highly variable chemical nature, but apparently characterized by some common properties. They are actually insoluble in water as non-polar substances (just try pouring oil into water to see how it will form circular structures called micelles). Lipids are important constituents of all animal and plant organisms, some of them, such as phospholipids, are present in all animal cells as the main components of the cell membrane, giving it the classic structure of a liquid mosaic and all its important chemical-physical properties that make it limited permeable. There are different types of lipid classification, here I will talk about the most famous and widespread, that is, we will see the difference between saponifiable and unsaponifiable lipids. But first we need to understand what the distinction is based on, which is not entirely clear; is largely based on the ability of a certain type of lipid to form soaps when treated with NaOH (caustic soda or sodium hydroxide), this property is largely determined by one factor, that is, the fact that the lipid contains acids or not (we will talk about this in more detail) , then, obviously, we can say that the saponifiable lipids contain ac. fats, but there is no unsaponifiability.
This name indicates a substance with a highly variable chemical nature, but apparently characterized by some common properties. They are actually insoluble in water as non-polar substances (just try pouring oil into water to see how it will form circular structures called micelles). Lipids are important constituents of all animal and plant organisms, some of them, such as phospholipids, are present in all animal cells as the main components of the cell membrane, giving it the classic structure of a liquid mosaic and all its important chemical-physical properties that make it limited permeable. There are different types of lipid classification, here I will talk about the most famous and widespread, that is, we will see the difference between saponifiable and unsaponifiable lipids. But first we need to understand what the distinction is based on, which is not entirely clear; is largely based on the ability of a certain type of lipid to form soaps when treated with NaOH (caustic soda or sodium hydroxide), this property is largely determined by one factor, that is, the fact that the lipid contains acids or not (we will talk about this in more detail) , then, obviously, we can say that the saponifiable lipids contain ac. fats, but there is no unsaponifiability.
Saponified faction
glycerides
When we talk about glycerides, we belong to a class of substances, including monoglycerides, biglycerides and triglycerides, and by the latter we mean esters of trivalent alcohol (glycerol) with 3 fatty acids. Triglycerides can be simple (glycerol is esterified by 3 equal fatty acids) or mixed (glycerol is esterified by at least one other fatty acid), hydrolysis of fatty acids from glycerol can occur chemically through the use of acids or bases (with strong bases such as NaOH, we will have not only the release of fatty acids, but also their salt formation in soaps) or in our body thanks to the help of specific enzymes, such as lipase.
Wax
Biochemically speaking, waxes are esters of monovalent acids with a large number of carbon atoms and higher fatty acids. This probably won’t mean anything to those who haven’t chewed on some organic chemistry, so let’s try to make the concept a little more digestible; to be exhaustive, I would have to write about the chemical-physical properties of alcohols, but for reasons of space, clarity of action and, above all, to preserve the mental health of those who have no idea what an atom is, I will avoid I’ll just say that each alcohol owes many of its chemical characteristics to the terminal hydroxyl group, that is, the -OH group, and an alcohol with one -OH group present in the chain is called monovalent (so you yourself will understand that divalent alcohol has 2 and one trivalent how glycerin has 3); Now let’s dwell on the word “esters”. It seems that we did not say anything with this word, and instead we opened another window for the second class of organic compounds, these ethers are actually products of the reaction between alcohol (or phenol, but let’s limit ourselves to the term alcohol, which is more than enough) with carboxylic acid, carboxylic acids (simplifying and contracting, as if there were no tomorrow) organic acids, which are precisely characterized by the presence of a carboxylic group, that is, COOH and that it is fatty acids that correspond to carboxylic acids, in fact, they also have a COOH group at the end of the chain. As a result, we have MONOVALENT ALCOHOL + AC. GREASE (long chain) = WAX this reaction that occurs between two molecules is called the esterification reaction; it is so called because an ester is formed, that is, wax, but in fact it is a banal condensation.
Steridi Attention, this is not a typo, not steroid steroids (you will find out about this later), another not entirely intuitive definition, steroids are esters of higher cyclic alcohols, if they are esterified with fatty acids, they are called sterols. Do not be discouraged by seemingly difficult concepts and definitions, I know that we seem to be talking about nothing, about very distant things drifting within the boundaries of the universe, in reality, you will soon realize that they are molecules that touch us closely, which influence every moment of our life and therefore must be heard at least once in a lifetime. So let’s talk briefly about sterols, they are a class of chemical compounds derived from sterol, that is, the famous polycyclic alcohol that we talked about earlier, on the carbon number 17 of this polycyclic alcohol we find a branched chain of fatty acids, and here is the revelation that make you fill your eyes and say the classic “aaaaann”; does the word coleSTEROLO tell you anything? Oh yes, cholesterol is zoosterol, which is a sterol of animal origin (we will soon find that cholesterol is one of the many “Romes” in our body that all roads lead to).
Phospholipids and Glycolipids
I feel deeply guilty and unworthy of respect for choosing to consider phospholipids and glycolipids together, but since they are molecules that need to be studied (yes, with a capital S) to be well understood, I will not be on them stop, this will save me time and research on old biochemical texts, and for you – an attempt to buy a new monitor after throwing the current one out of the window. However, we are talking about phospholipids, they can be divided into phospholipids (or phosphatides) and sphingolipids (or sphingomyelin), which we start with real phospholipids, each phospholipid structure comes from the so-called phosphatidic acid, this acid in turn comes from the glycerol molecule esterified 2 fatty acids and a phosphoric acid molecule, this phosphoric acid, once reacted with glycerol, still has two groups (called acidic functions) with which it can additionally react, forming a phospholipid, with an amino alcohol (which, obviously, we will not deal with ); but in essence, what are these phospholipids biologically used for? The role of these molecules is very important, in fact, they are extremely widespread in nature, first of all, they are the main components of animal cell membranes, which should be enough to give an idea of the basilicity of these molecules, but this is not finished yet, for example, here. phosphatidylcholine (yes you, this supplement that you know as a cortisol modulator is a phospholipid) provides other compounds such as arachidonic acid, very important in the response to inflammation, not only that they are also very important molecules for transmitting nerve impulses , in fact, the axons of neurons are covered with a special membrane called myelin guarine, which is partially composed of phospholipids and provides efficient transfer of electrical potential.
Now let’s move on to glycolipids, they consist of an amino alcohol (sphingosine), a fatty acid and a hexose, that is, a sugar with 6 carbon atoms (as glucose can be), but usually it is galactose, their biological function is not entirely clear, it is known that they are extremely present in the brain at the white matter level, in the myelin sheaths of neurons, as well as at the level of leukocytes and erythrocytes, and are believed to play an important role in the formation of antigens, therefore they will be important for the recognition of substances. exogenous and maintain cellular balance.
Unsaponifiable fraction
Steroids
As I write, I feel that the bubble burst from the readers’ nose, those who snored now widened their eyes and returned to the living, and everyone read the word “steroids”, yes, for you brave ones, who have read this far, it is time to understand something about these molecules (of course, always from a biochemical point of view). The term steroids denotes a large group of substances that have different biological roles, but share a common basic structure. This structure of cyclopentanoperidrofenanthrene, steroids can be further divided into other classes of substances; sterols and steroids, bile acids, sex hormones, hormones of the adrenal cortex, aglycones-glucosides (glucoside derivatives that have a cardiokinetic effect, that is, they can modulate the heart rate).
All the numerous properties of steroids significantly depend on the assumed spatial configuration of their main structure, cyclopentanoperidrophenanthrene, as well as on the chemical nature of any side chains present in the molecule, the presence of certain functional groups such as hydroxyl (-OH), However, when we say about steroids, the first molecule we need to talk about is necessarily cholesterol (… what did you expect? …), this molecule is important not only because it is able to stiffen our cell membrane, without a wall, unlike plant (This is why cholesterol is not present in plants .. it is simply not needed), but it is also a precursor to many other molecules such as sex hormones, bile acids (tauroleic and glycolic acids) and vitamin D. Cholesterol is also present in the white matter of the brain, which accounts for 14% from dry weight and in smaller quantities even in gray. In human blood under normal conditions, it is present (associated with lipoproteins) in an amount of 180-230 mg per 100 ml, this molecule is partially of exogenous origin, is introduced into the diet, especially through meat, but it is also synthesized by endogenous liver studies (both in vivo and and in vitro) show that synthesis can occur from very simple molecules such as acetate.
Bile acids are the main product of cholesterol catabolism, in fact, it is used to produce about 80/90% of exogenous cholesterol, since we all know that these acids in the form of 2 tauro acid salts and sodium and potassium glycocolate are the main components of bile (produced by the liver) and play a fundamental role in the digestion of fats, in particular, their emulsifying activity divides fats into microscopic lipid droplets that are easily attacked by digestive enzymes such as pancreatic lipase.
I decided to rid you of the terpenes (don’t laugh if they haven’t chosen a name) as I don’t think they are important for the purposes of the next discussion.