Let’s face it, BodyBuilding is not only a subculture, but also a science. It is an interdisciplinary science that includes physiology, biology, endocrinology, metabolism, cellular physiology, genetics, molecular biology and, we must not forget, pharmacology. The list of scientific sectors related to BodyBuilding is very long.
Personally, I consider the BodyBuilding competition to be a kind of exhibition after experimentation. When I reflect on the current situation of professional bodybuilding, I cannot help but notice that the current specialists (or at least from abroad) manage to change the threshold of improvement, always exceeding their limits: even if I am not a lover of “bigger and better” far preferring “correct for the subject”.
Whenever I find myself watching a high-level competition closely while also being a BodyBuilder trainer, I can’t help but think all the time about the energy, food, genetic manipulation and medications needed to build fitness , which can dramatically overcome the genetic limit. Following the athletes, allowing them to overcome their limits, is a manifestation of dedication, the correct application of knowledge. Using drugs and genetic manipulation to enlarge and shrink the human body is not an exercise in scientific awareness. It is an expression of the human understanding of the scientific sectors mentioned earlier in order to take a kind of control over individual genetics. Unfortunately, the most important scientific journals do not recognize BodyBuilding as a real sector of scientific research. So, for now, in order to achieve our goals, we coaches and our BodyBuilders need to be happy with extrapolating the right information from leading research areas.
With the advent of peptides, this “experiment” on Body Builders reached incredible levels until the 1990s. In this article I will present a holistic picture of the “fantastic trio” of the world of peptides, trying to fully explain their nature and actions. Some information recently extracted from academia and applied to BodyBulding will be presented. We will focus on the proper use of growth hormone (GH), insulin-like growth factor 1 (IGF-1) and insulin for muscle building. This information will be presented in such a way as to describe how these growth factors can be incorporated into medications.
I will not advise, I do not report all of this for general prescription purposes, but I do it for culture and knowledge. against damages disinformation “food” and “anti-doping”.
Axis GH / IGF-1
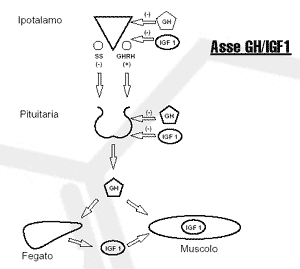 GH levels in the body are tightly regulated by numerous chemicals that include macronutrients, neurotransmitters, and hormones. The signal to increase the level of GH in the body is part of the hypothalamus. There, two peptide hormones work together to increase or decrease the production of GH by the pituitary gland. These hormones are somatostatin (SS) and growth hormone releasing hormone (GHRH). Somatostatin acts in the pituitary gland to decrease the production of growth hormone. GHRH acts in the pituitary gland to increase GH production. Together, these two hormones pulsate the level of GH that circulates in the body. There are many factors that can affect this delicate balance. First, GH is subject to negative feedback in response to its release. GH, like IGF-1, returns to the hypothalamus and pituitary gland to increase SS release, thereby decreasing GH release. GH can also act in an autocrine and paracrine manner (that is, by acting on source cells and surrounding cells without having to enter the bloodstream) in both the hypothalamus and the pituitary gland.
GH levels in the body are tightly regulated by numerous chemicals that include macronutrients, neurotransmitters, and hormones. The signal to increase the level of GH in the body is part of the hypothalamus. There, two peptide hormones work together to increase or decrease the production of GH by the pituitary gland. These hormones are somatostatin (SS) and growth hormone releasing hormone (GHRH). Somatostatin acts in the pituitary gland to decrease the production of growth hormone. GHRH acts in the pituitary gland to increase GH production. Together, these two hormones pulsate the level of GH that circulates in the body. There are many factors that can affect this delicate balance. First, GH is subject to negative feedback in response to its release. GH, like IGF-1, returns to the hypothalamus and pituitary gland to increase SS release, thereby decreasing GH release. GH can also act in an autocrine and paracrine manner (that is, by acting on source cells and surrounding cells without having to enter the bloodstream) in both the hypothalamus and the pituitary gland.
Neurotransmitters also affect hypothalamic GH levels. This neuroendocrine control remains to be elucidated, but several factors are already clearly at work (see table). As you of course know, dietary and metabolic factors also affect GH levels. A drop in blood sugar, such as that occurs during exercise or during sleep, causes an increase in growth hormone secretion. Protein-rich foods increase the secretion of growth hormone. Certain amino acids, such as L-arginine, appear to increase GH by decreasing the release of SS from the hypothalamus. The vitamin niacin has also been shown to increase activity-induced GH excretion by 300-600% ( Murray, 1995 ).
In this particular study, 4 different tests were conducted in which 10 subjects cycled at 68% of their maximum VO2 for 120 minutes, followed by 5.6 km. Every 15 minutes of physical activity, subjects consumed 3.5 ml per kg of lean body weight from one of 4 drinks:
- Water Placebo (WP)
- WP + 280 mg niacin 1.1 (WP + NA)
- Drink with 6% carbohydrates and electrolytes (EC)
- CE + NA
Nicotinic acid supplementation (WP + NA and CE + NA) attenuated the increase in free fatty acids (FFA) associated with WP and CE; in fact, nicotinic acid supplementation effectively prevented increases in FFA beyond resting values. Low FFA levels with niacin was associated with 3-6-fold increases in human growth hormone concentration during physical activity. However, the question remains whether this significant but temporary increase in GH has a greater impact on training. This could create a higher storage capacity for glycogen, but beyond that we don’t know for sure.
Reducing calories dramatically lowers blood IGF-1 levels and at the same time increases GH release. This mechanism effectively assists the subject to adapt metabolically without the need to perform anabolic actions that could potentially accelerate fasting. It is important to understand that GH can be anabolic or catabolic. When nutrient intake is high, GH secretion is increased, producing even higher levels of IGF-1, IGFBP3, and insulin. Under these conditions, the main role of growth hormone is to enhance anabolism by local growth factors such as IGF-1 and insulin.
Even when nutrient intake is low, GH increases, but this time there is no concomitant increase in IGF-1, IGFBP3 or insulin. In these (low-calorie) circumstances, GH acts as a catabolic hormone that increases the use of fat for fuel, thereby conserving glucose in the body, but not affecting muscle building. This GH / IGF-1 axis behavior is part of what makes it difficult to build muscle during a reduced calorie diet. It should be noted that during the diet, IGF-1, produced locally in skeletal muscle, responds to exercise normally. This makes heavy lifting a must when trying to prepare for a drug-free race. Obviously, we are talking about endogenous situations, and not about changes caused by exogenous hormonal injections.
GH: functions and applications in bodybuilding
Before using any substance (medication or simple OTC supplement), you should have a basic knowledge of how the supplement, hormone, or medication works to build and / or maintain muscle mass. Knowing how a hormone works in the body is essential to making decisions and managing usage patterns. Without this knowledge, you will undoubtedly lose a lot of money and, more importantly, risk your health.
GH (growth hormone), also known as somatotropin (STH), growth hormone, growth hormone, is a peptide hormone secreted by the adenohypophysis, consisting of 191 amino acids with a weight of 22.005 Da. Its main function is to stimulate the development of the human body, promoting the growth and mitotic division of cells in virtually all tissues of the body. For a long time, it was believed that GH exerts an anabolic effect on peripheral tissues through IGF, also known as somatomedins (“growth mediators”). Binding proteins play an important role in mitigating the anabolic effects of both GH and IGF-1. IGF-1 is controlled by at least 6 different binding proteins and there may be others that can be found. Today, there are several theories about how GH induces tissue growth. The first theory is called the somatomedin hypothesis ( Daughaday, 1972 ):
- The somatomedin hypothesis states that GH is released by the pituitary gland and then travels to the liver and other peripheral tissues, where it triggers IGF synthesis and release.
IGFs are so named because of their structural and functional similarities to proinsulin. This hypothesis suggests that IGFs function as endocrine growth factors, which means that once released by their producing tissues, in particular the liver, in which case they travel in the blood to the target tissues. Many studies have shown that systemic IGF-1 infusions result in normal growth in GH-deficient animals. The effects were similar to those seen after the administration of GH.
Interestingly, other studies followed that show that IGF-1 is a much lower endocrine growth factor as it takes almost 50 times more to have the same effects as GH ( Skottner, 1987 ). Recently, rHIGF-1 has become much more widely available and is now approved for the treatment of HIV-related degenerative syndrome. This greater availability has made it possible to experiment with this hypothesis in humans. Studies in subjects with GH insensitivity (Laron’s syndrome) consistently support the somatomedin hypothesis ( Rank, 1995) ; Savage, 1993 ).
The second theory of how GH induces anabolic effects is called the effector double theory ( Green, 1985 )
- GH alone has anabolic effects on body tissues without the need for IGF-1.
This theory has been supported by studies in which GH was injected directly into growth plates. Additional evidence to support this theory is presented in genetically modified strains of guinea pigs. When comparing guinea pigs with genetically high GH values and guinea pigs with genetically high IGF-1 values, those with high GH values are larger. Someone has used this evidence to support the dual effector theory. Interestingly, when an anti-IGF-1 antiserum (destroys IGF-1) is injected with GH, all of the anabolic effects of GH are reversed.
The somatomedin theory and the dual effector theory are not very different. It simply claims that GH can grow without IGF-1. Based on research, I tend to believe in the somatomedin theory. The question only becomes important when deciding whether to use GH alone or combine it with IGF-1 or insulin.
Based on the available information, there are three important mechanisms by which GH grows ( Spagnoli, 1996 ):
- The effects of GH on bone formation and organ growth are mediated by the endocrine action of IGF-1.
As mentioned in the somatomedin hypothesis, GH secreted by the pituitary gland causes greater production and release of IGF-1 in the general circulation. IGF-1 then travels to target tissues such as bones, organs and muscles, causing anabolic effects.
- GH regulates IGF-1 activity by increasing the production of binding proteins (in particular IGFBP 3 and other important proteins called acid-labile subunits), which increase the half-life of IGF-1 from minutes to hours. The circulating proteases then act to break down the protein / hormone binding complex, releasing IGF-1 in a controlled manner over time.
GH can also induce the production of IGFBP-3 in target tissues by increasing its local effectiveness.
- In target tissues, IGF-1 has not only endocrine, but also paracrine / autocrine effects.
This means that when GH reaches the muscles, the muscle cells increase the production of IGF-1. This IGF-1 can then travel to neighboring cells (especially satellite cells), causing growth and increased regenerative capacity of cells that have not been seen by GH. This is according to the dual effector theory.

It is interesting to note that babies produce 2UI GH “peaks” four to seven times a day for four / five non-consecutive days for two / three weeks (during peak growth). This equates to 32-70 IU in just four to five days. The pituitary gland of a healthy adult only releases 0.5-1.5 IU per day. Until the mid-1980s, the only available form of exogenous GH was produced by removing the pituitary gland from corpses and crushing it. The GH was then extracted and purified through a series of expensive procedures, packaged and sold by prescription only for use by children with growth problems. For this and other reasons, GH was closed to Arnold or Zane’s Old School. In 1987, this form of GH was associated with a fatal brain disease called Creutzfeldt-Jakob disease and was removed from the market.
Then Genetech came in and launched synthetic GH. The first synthetic GH was produced by genetically modifying the cells of transformed guinea pigs. Natural GH, as we well know, has a sequence of 191 amino acids, while GH Protropin, produced by Genetech, has 192 amino acids in its sequence. This can stimulate the body to produce antibodies to GH, which deactivate it. Most synthetic products now contain the normal 191 amino acid sequence. There are a dozen of them on the market today. However, in some subjects, the body produces antibodies after administration of exogenous GH, denying its use. GR, when used correctly, is known to be a genetic equalizer when used for this purpose.
Obviously, the launch of synthetic GH allowed athletes (especially BodyBuilder) to be able to incorporate it into their preparations. The reason athletes turned to exogenous GH (already mentioned above) is simple and stems from three effects any athlete wants:
- GH helps the body burn more fatty tissue by promoting the release of fatty acids for use as energy .
Normally the body uses equal amounts of fat and calories at rest.
When the endocrine system senses low circulating glucose, the hypothalamic-pituitary axis (HPA) responds by releasing GH. GH triggers (through a series of enzymatic / chemical reactions) the release of fatty acids from fat stores to meet metabolic energy needs. It has been well documented that administration of exogenous GH also has the same effect.
- GH has a very strong anabolic effect. When exercising its anabolic effects, it can cause both muscle hyperplasia (increase in the number of muscle cells) and muscle hypertrophy (increase in muscle fibers).
This change in the number of muscle cells is permanent, so it means more cells will grow. GH also has an anabolic effect on soft tissues such as tendons, cartilage, and other connective tissues. This means that, thanks to stronger connective tissue, old lesions heal and strength increases, and at an accelerated rate: unfortunately, this anabolic action leads (dose / time dependent) to neuropathy. It is well known that GH is a potent anti-catabolic agent (protein defense). This has allowed (among other things) modern bodybuilders to maintain or even increase muscle mass during periods of low calorie intake (defining / pre-race phases).
- GH is converted to IGF-1 in the liver.
The body has a limited ability to convert excess GH to IGF-1 unless other hormones are also high. Insulin, thyroid hormones (T4 / T3), gonadotropins, AAS, as well as estrogens and corticosteroids all play an important role in the positive effects of GH. Thus, they have also been added by some athletes to achieve hormonal “right relationships”. In order for the liver to convert high levels of GH to IGF-1 several times a day, triggering a high level anabolic response, it was often said that thyroid hormones and insulin had to increase as well. Synthetic T3 was also considered the best choice because GH suppresses the secretion of the natural hormone T3. The use of * fast (Humulin-R) insulin allowed athletes to synchronize exogenous insulin activity with the active GH period at moments of optimal absorption, such as, for example, just rising and in the first hours after training. The result is less fat storage and a greater anabolic response.
( * The use of insulin is very dangerous. If used improperly, it can lead to coma and If you want to use it, try under the close supervision of a doctor or trainer with adequate medical skills. )
GH Dosage and Administration
The question of the “ideal” dose of growth hormone is complex. For the treatment of stunting, growth hormone manufacturers recommend 0.3 IU per week for every 0.5 kg of body weight.
Therefore, for a bodybuilder weighing 106 kg, 64 IU per week is required, which corresponds to a daily dose of about 10 IU. However, even 2-3 IU per day gave good results for 6-8 weeks when other hormonal needs were met. Even short cycles with high doses produced superior effects in athletes who knew how to manage everything “smartly”. One of the positive aspects of short and intense GH cycles is that there is no significant increase in somatostatin: however, somatostatin is readily reduced with the use of L-arginine and its analogues. The range of GH doses used by athletes on average is approximately 2 IU to 16 IU per day (1 mg = 2.7 IU GH).
There are two methods of administering GH: subcutaneous or intramuscular injections. Most athletes choose subcutaneous injections. If you look at the clinical information provided by various GH manufacturers or various studies available on the web, you will see that the bioavailability of GH given by subcutaneous or intramuscular injection is very similar, as are the mean plasma concentrations after administration. Although, in the case of subcutaneous injections, this is about 10% more due to slightly faster absorption of GH than when injected intramuscularly (half-life is about 3-4 hours injected subcutaneously and about 4-5 hours injected intramuscularly) .
However, since the absorption rate is slightly higher and plasma levels are slightly higher when injected subcutaneously, this will not necessarily result in higher IGF-1 levels; there is no significant pharmacological difference between the two methods. However, it should be said that in the case of multiple injections, many athletes and trainers have noticed better results with subcutaneous administration.
When GH is used in a protocol that includes the use of insulin, it is important that the distance between the GH and insulin injections is about an hour. In addition, if GH is administered only twice a day, it is advisable to avoid administration at times of strong natural GH release, such as in the early morning, after exercise, and just before bedtime. This is if GH was used without insulin. It is fair to remember that GH increases the growth of type I fibers more than type II fibers. Co-administration of strong androgens (such as trenbolone) allows you to “convert” type I fibers to type 2 fibers for strength and bulk. This is an example of the long-term synergy created by using GH.
Some interesting points about GH / insulin synergy:
- Insulin promotes cellular absorption of about half of the amino acids for repair and growth, while GH promotes absorption of the other half.
- Insulin increases the conversion of T-4 to T-3, while GH decreases the conversion of the liver.
- Insulin is hypoglycemic – GH is hyperglycemic.
- Insulin stores fat – GH stores fat.
Using GH without AAS or insulin is anti-catabolic, but not anabolic. The use of GH alone does not significantly increase the contractile proteins of muscle fibers. This is precisely because GH does not have the ability to induce the absorption of all essential amino acids, and therefore most of the growth occurs in structural proteins. As mentioned above, since GH lowers T-3 levels. In addition, by decreasing protein synthesis, not administering triiodothyronine is a poor choice. As almost everyone knows, a huge amount of AAS and counterfeit drugs are circulating around the world. also synthetic GH. When bought on the black market, many simply bought a pregnancy test to see if the GH was true. After mixing the vial of (presumably) GH, a drop or two is placed at the test point. If the result is “positive”, you are faced with simple gonadotropin uk (hCG).
When you can (if money allows), be sure to buy GH in pharmacies such as Saizen or Genotropin. If you can’t get pharmaceutical GH, you can roll back to Hygetropin, obviously making sure it’s valid.
The side effects found when using GH are as follows:
- enlarged kidneys
- Enlarged heart
- High blood pressure
- Diabetes
- Hypothyroidism
- Acromegaly
- Neuropathy
These are rare side effects (with the exception of neuropathy), usually due to extremely high doses and very long cycles. Unfortunately, problems arise when they become urgent.
hGH snippet 176-191
There is a variant of GH called hGH Fragment 176-191. The researchers found that if the original peptide binding at the C-terminal region is truncated, then the fat loss associated with classic GH is isolated. Thus, the resulting peptide was found to have the ability to increase fat loss 12.5 times better than classic GH. The hGH fragment 171-191 is therefore used by those BodyBuilders who do not seek the anabolic properties of GH, but have amazing fat loss properties.
As mentioned, the hGH fragment 176-191 has incredible ability to regulate fat metabolism, with the addition of not causing negative side effects on insulin sensitivity. In fact, it also inhibits lipogenesis; and this means that it has the ability to stop the synthesis of fatty acids and other lipids. Since it does not compete for GH receptors, some studies have shown that the hGH fragment 176-191 does not cause hyperglycemia.
To benefit from the use of the hGH fragment, users take doses of about 500 mcg per day. This can be done by dosing 250 mcg in the morning before training and 250 mcg before lunch, or 250 mcg before bed. Users should consider timing of injections and their meals. Optimally, the hGH fragment 176-191 is administered on an empty stomach or exclusively with protein flour. As with other peptides, the hGH fragment is not effective when taken simultaneously with food containing carbohydrates and sugars.
Typically, the hGH Fragment is administered with GH secreting agents such as GHRH-6. When using the hGH fragment, there are no problems with thyroid hormones, glucose sensitivity, tingling sensations, or problems with the carpal tunnel. Side effects most commonly associated with hGH fragment 176-191 are minor but may include: redness or pain at the injection site, and excessive sleepiness or lethargy.
Recently, several peptides with the potential for GH secretion have been depopulated in the amateur bodybuilding world (see also Ibutamoren). There are different types and with different side effects; Among the most used are GHRP-2, GHRP-6, Ipamorelin and CJC 1295. Personally, I find these peptides to be a good choice only for those who cannot afford GH or for beginners.
IGF-1: Features, Options and Applications in Bodybuilding
Insulin-like growth factors, also known as IGFs (insulin-like growth factor) or somatomedins, are a group of peptide hormones with anabolic properties that are produced by the liver under the influence of growth hormone (GH). ) is produced by the pituitary gland. There are two isoforms:
- IGF-1 (somatomedin C or SM-C): It is maximal during puberty and decreases with age. This is strictly up to GH.
- IGF-2 (somatomedin A or SM-A): It is present primarily in fetal life and is only partially dependent on growth hormone.
We’re obviously focusing on IGF-1 here.
To understand how IGF-1 works, you need to understand how muscle grows. The ability of muscle tissue to constantly regenerate in response to physical activity makes it unique. Its ability to respond to physical / mechanical stimulus depends largely on what are called satellite cells. Satellite cells are muscle progenitor cells. They can be considered “promuscular” cells. These are the cells that are located above and around muscle cells. These cells remain dormant until challenged by growth factors such as IGF-1.
After that, these cells divide and genetically transform into cells that have a nucleus identical to the nucleus of muscle cells. These new muscle nucleus satellite cells are required, if not needed, for muscle growth. Without the ability to increase the number of nuclei, the muscle cell will not grow and its ability to heal itself will be limited. The explanation for this is quite simple. The cell nucleus is where all new growth projects are born. The larger the muscle, the more nuclei are required to maintain it. In fact, there is a “volume-core” relationship, from which it is impossible to escape. Whenever a muscle grows in response to functional overload, there is a positive correlation between an increase in the number of myonuclei and an increase in fiber cross-sectional area (CSA). When satellite cells cannot donate new nuclei, the overworked muscle does not grow ( Rosenblatt, 1992 and 1994 ; Phelan, 1997 ).
You see, an important factor in unnatural muscle growth is the activation of satellite cells by growth factors such as IGF-1. IGF-1 stimulates both proliferation (increase in the number of cells) and differentiation (transformation into specific muscle nuclei) in an autocrine / paracrine manner even if it primarily stimulates differentiation. The dual effector theory also supports this. In fact, you can inject IGF-1 into a muscle and watch it grow! Studies have shown that when administered topically, IGF-1 increases satellite cell activity, muscle DNA content, muscle protein content, muscle mass and muscle cross-sectional area ( Adams, 1998 ).
Researchers are currently discovering a signaling pathway through which mechanical stimulation and IGF-1 activity induce all of the aforementioned changes in satellite cells, muscle DNA content, muscle protein content, muscle mass and muscle cross-sectional area. This research comes from research done to explain cardiac hypertrophy. This includes a muscle enzyme called calcineurin, which is a phosphatase enzyme activated by high intracellular calcium ion concentrations ( Dunn, 1999 ). Note that overworked muscle is characterized by chronically high intracellular calcium ion concentrations. Another recent study showed that IGF-1 increases intracellular calcium ion concentrations, which leads to activation of the signaling pathway and subsequent hypertrophy of muscle fibers ( Semsarian, 1999 ; Musaro, 1999 ).
In general, the researchers who participated in these studies gave this explanation:
IGF-1, in addition to activating calcineurin, induces the expression of the transcription factor GATA-2, which accumulates in a subset of muscle nuclei, where it is associated with calcineurin and a specific dephosphorylated form of the nuclear factor-activated transcription factor cells T or NF-ATc1.
Therefore, IGF-1 induces signaling and activation of GATA-2 mediated by calcineurin , a hallmark of skeletal muscle hypertrophy that interacts with specific NF-ATc isoforms to activate genetic expression programs that result in greater the synthesis of contractile proteins and muscle hypertrophy.
A side effect of taking C-17 methylated AAS is also increased production. natural IGF-1 from the liver. Since IGF-1 receptors are present in muscles and organs such as the heart, spleen, small intestine and kidneys, the higher the concentration of the receptor, the greater the effect it has on the organs.
IGF-1 recombinant (genetically modified) is effective in intramuscular injection because it induces localized growth. This is the most commonly used method. The half-life of the drug is about 10 minutes, and if it binds or has been bound to IGF-BP3 (IGF-1 binding protein), the half-life increases to about 12 hours.
Insulin and / or GH with IGF-1 because that the latter blocks the production of natural GH and GH causes insulin resistance. IGF-1 is often considered proinsulin because it fights insulin resistance and interacts with insulin. However, this is an imprecise definition.
IGF-1 can have all the side effects of using GH or insulin with an additional disadvantage: GI tract growth . This is because there are many more IGF-1 receptors in the gastrointestinal tract than in skeletal muscle. The latter have several receptors for GH. This explains much of the swelling seen on several occasions in high-level bodybuilding competitions. IGF-1 has been used clinically in children at doses greater than 3-7 mg per day. That is 3000-7000 mcg per day! No negative side effects were experienced or expected. At BodyBuilding, average doses are clearly lower, ranging from 60 to 1000 mcg per day, divided into intramuscular injections performed on trained muscles. Needless to say, very noticeable local growth is observed with IGF-1. It is important to note that IGF-1 can cause hypoglycemia and therefore it is important to use a blood glucose test kit.
IGF-1 options: Des (1-3) and LR3
When a chemist removes the last three amino acids in the chain (N-terminal tripeptide), IGF-1 becomes Des (1-3) , which is 1000% more anabolic. The reason is very simple: most of the circulating IGF-1 is inactive because it is bound to another protein called IGF-1 3-binding protein, or IGF-1-BP3. Since bound hormones cannot enter their receptor site to stimulate it, circulating and muscle IGF-1 cannot elicit an anabolic stimulus. But when IGF-1 is modified and converted to Des (1-3), the IGF-1-BP3 binding protein cannot bind to it and is fully active.
Another reason Des (1-3) is for its unique ability to bind to lactic acid-modified IGF-1 receptor sites: when we exercise, we use energy substrates such as carbohydrates to produce cellular ATP. When cells pass through this ATP pathway, lactic acid is a byproduct. This is, of course, the main cause of the burning sensation that is felt when doing intense series or high repetitions. Thus, the accumulation of lactic acid is called acidosis and destroys the shape of some receptor sites for a while.
Therefore, some anabolic / anti-catabolic hormones have difficulty (even unbound IGF-1) to bind to their respective receptor site and stimulate a response. This is not the case with Des (1-3), which binds to the IGF-1 receptor site even after acidosis. Des (1-3) is unbound, more than 10 times more potent than IGF-1, and frees up receptor site entries. Unfortunately, his active life is only a few minutes. It is interesting to note that the body is capable of producing Des (1-3). When an athlete trains, lactic acid builds up in muscle tissue. As we know, residues of GH / IGF-1 from previous workouts and other metabolic factors are always present in blood and tissues (including muscles). Burning lactic acid stimulates IGF-1 / GH production from current and previous workouts. Unfortunately, lactic acid breaks down some of the IGF-1 present in trained muscles. But this is positive.
Lactic acid cuts (truncates) the last 3 amino acids of the 70 “parts” of the amino acid chain of the surviving IGF-1 and creates Des (1-3). Consequently, acidosis increases the production of GH / IGF-1 by the liver, locally binds IGF-1 in trained (burned) muscle, destroys part of IGF-1 and converts another part of it into Des (1-3). For several years, the synthetic version has been increasingly found on the black market.
Average doses of Des (1-3) used vary from 50 to 150 mcg several times a day (post-workout) in injections localized to trained muscles. Since Des (1-3) has a short half-life (20-30 minutes), receptor desensitization has not been detected.
There is another IGF-1 form, similar to the above Des (1-3): LR3. Like Des (1-3), LR3 is chemically modified so that it does not bind to the transport proteins IGF-1-BP3. But there is another thing: although Des (1-3) has an active life of only a few minutes, LR3 has a half-life of 20-30 hours! In a nutshell, LR3 is a long-running version of the classic IGF-1. However, it is less powerful than Des (1-3). Due to its long half-life, LR3 is often injected into a “specific” site due to the fact that growth is enhanced in general rather than locally. LR3 has the ability to prevent glucose from entering cells, which in turn leads to metabolic constriction, which causes the body to burn fat rather than glucose. Average doses used for LR3 are about 50-150 mcg per day every day for a maximum period of 40 days (or better 4 weeks), since receptor desensitization occurs around this time period.
It’s important to understand that long-term negative side effects have not yet been well analyzed. Anything that can change genetics also has negative potential .
Anabolic androgens and increased GH and IGF-1 levels
For now, I would like to deepen the question, namely stimulation of GH and IGF-1 with AAS. While this isn’t really an article about androgens per se, androgens play an important role in modulating GH when trying to increase muscle mass. To understand this relationship, we must go back to the difficult years of puberty.
During puberty, the body becomes challenged in its ability to precisely regulate GH levels, creating higher levels of GH, IGF-1 and insulin. This is combined with the strong testosterone production that characterizes puberty. Research has shown that this jam is caused by testosterone aromatization and some of the direct actions of androgens.
In a recent study by Friborg The effects of testosterone and stanozolol on stimulating GH release have been compared. Testosterone enanthate (only 3 mg / kg per week) increased GH levels by 22% and IGF-1 levels by 21%, while oral stanozolol (0.1 mg / kg per week) did not affect GH or IGF-1 levels. A couple of notes on this study. It only lasted 2-3 weeks, and while stanozolol did not affect GH or IGF-1 levels, it did have a similar effect on urinary nitrogen levels. Urine nitrogen is dense with disorienting variables when used to measure skeletal muscle anabolism and / or catabolism and, therefore, should not be considered an accurate indicator of skeletal muscle growth. The use of labeled amino acid tags or 3-methylhistidine is a much more accurate way to determine the efficient synthesis and degradation of contractile proteins. However, this study may partially explain the observation that many bodybuilders do not respond to testosterone-based foods with complete estrogen blockade. It is also true that administration of oral AAS methylated at C-17 induces methylation-induced liver stress, increasing IGF-1 production in the liver. Typically, the dosages required to significantly increase the production of IGF-1 in the liver exceed 0.1 mg / kg body weight in an earlier study.
Too much Cytadren, and especially Arimidex, prevents gynecomastia and excess water retention, but also decreases muscle gains through less dramatic increases in GH and subsequent lower IGF-1 levels. In vitro, some androgens have also been observed to increase the sensitivity of satellite muscle cells to fibroblast growth factor and IGF-1.
Remember that satellite cells are essential for cell growth. Bovine satellite muscle cells were able to fuse 20% faster with trenbolone and estradiol treatment. One might think that not only trenbolone, but also estradiol caused a significant increase in nutritional efficiency and muscle growth by increasing the production of GH and IGF-1 in both the liver and cells. muscles. It is clear from these studies that IGF-1 is required to maximize the anabolic activity of androgens. This means that androgens that increase GH production (that is, those that aromatize) are likely to cause the most sustained and rapid increases in muscle mass.
It is also true that the use of oral AAS methylated at C-17 has its own Cause of methylation-induced liver stress, increases the production of IGF-1 in the liver. Obviously, the usual doses required to significantly increase IGF-1 production in the liver are in excess of 0.1 mg per kg of body weight in the study study.
Insulin: functions, options and applications in bodybuilding
Insulin is a peptide hormone with nonspecific anabolic properties, produced by the beta cells of the islets of Langerhans inside the pancreas; it consists of two chains connected by two sulfide bridges: chain A of 21 amino acids and chain B of 30 amino acids. Its most famous function is to regulate blood glucose levels by lowering blood glucose levels by activating various metabolic and cellular processes. It also plays an important role in proteosynthesis (protein synthesis) along with the aforementioned hormones that synergistically participate in this process: the GH / IGF-1 axis and testosterone. Insulin stimulates the flow of glucose into the cytosol of insulin-dependent organ cells by binding to the external receptor of the cell membrane.
This function is possible due to the interaction of insulin with its receptor present on the cell membrane, which promotes the phosphorylation of three tyrosine residues of the IRS-1 peptide located in the cytoplasm. Phosphorylated peptide promotes phosphorylation of phosphatidylinositol-3-kinase (PI3K) in phosphatidylinositol-3,4,5-triphosphate (PIP3), which phosphorylates phosphatidylinositol-3-kinase (PI3K) and phosphorylates phosphatidylinositol-3,4,5-triphosphate … in turn, the insulin-sensitive protein PKB. This protein renders the enzyme glycogen synthase kinase inactive, which is responsible for the inactivity of glycogen synthase. This enzyme, stimulated in this way by insulin, facilitates the formation and elongation of glycogen molecules in the liver and skeletal muscle by combining glucose monomers. At the same time, it interferes with the breakdown of glycogen by glycogen phosphorylase, depriving it of its phosphate group using the enzyme phosphatase.
Insulin deficiency or cellular resistance to it causes a deficiency of glucose-6-phosphate, which is necessary for the intracellular process of glycolysis, which synthesizes pyruvate, starting with glucose. Oxaloacetate together with acetyl-CoA forms the basis of the Krebs cycle. The excess acetyl-CoA, no longer suitable for citrate condensation, is intended for the ketogenic pathway for energy production by releasing CoA-SH and ketone bodies responsible for diabetic ketoacidosis.
Its antagonistic hormones are cortisol (the hormone underlying insulin resistance), adrenaline, glucagon, aldosterone, and GH. The hormones that improve its action are testosterone, insulin-like growth factor and, to a lesser extent, estrogens (they stimulate the synthesis of a protein called transcortin, which binds and inhibits cortisol).
The two chains are derived from a single polypeptide from which peptide C is excised, a short protein fragment apparently devoid of physiological function that, when secreted with insulin, is a useful indicator of islet functionality.
Insulin also performs other equally important functions, because it stimulates mitosis, growth of muscle and bone mass; unlike other anabolic hormones, it also stimulates the growth of fat mass; raises LDL cholesterol levels.
In the central nervous system, especially in the neurons that make up the hypothalamic center of satiety, we find insulin receptors. In fact, in the brain, this hormone does not regulate glucose metabolism, but regulates food intake, as it reduces hunger.
Consequently, when a person has low insulin levels (for example, in a diabetic), they tend to eat more than they should since insufficient insulin action does not make him feel full, it is easier to become obese.
Usually when the properties of the hormone insulin are mentioned, the function of lowering blood sugar (glucose) levels is mainly treated by transporting them to certain tissues that act as storage sites or storage sites (insulin tissues). dependent), that is, skeletal muscle tissue, heart and adipose tissue, as well as other tissues in respect of which it has an indirect effect on glucose uptake. In fact, insulin interferes anyway with the simple purpose of “feeding” these tissues, and after the introduction of other nutrients such as proteins (or amino acids and peptides) and lipids, and not only with the task of managing any excess blood sugar.
Insulin plays a role in protein synthesis in synergy with GH (or somatotropin), IGF-1 (or somatomedin c), and testosterone. After the introduction of proteins, the resulting amino acids are partially used for protein synthesis and, as a rule, for growth. Many amino acids can stimulate insulin, but their insulinogenic strength varies depending on, for example, glucose levels and mixing with it (see insulinogenic amino acids).
Mixed amino acids and pure protein flour induce insulin production, but less than pure carbohydrate flour. The secretion of this hormone after protein meal promotes the absorption and storage of amino acids in the form of muscle proteins and counteracts proteolysis (protein catabolism), a process that promotes the use of amino acids for energy for gluconeogenesis, mainly during fasting.
Some researchers say that insulin’s primary role is to reduce protein catabolism, while actually playing a minor role in protein synthesis, although some studies have shown the opposite. Other evidence suggests that an increase in insulin without a concomitant increase in amino acid availability leads to a decrease in protein synthesis due to a decrease in the concentration of amino acids in the blood. Instead, amino acids derived from dietary proteins appear to have their primary effect of increasing protein synthesis with minimal impact on protein catabolism, although not all studies have confirmed this effect.
Proteins stimulate the secretion of growth hormone and insulin. Both, in turn, promote the production of IGF (insulin-like growth factor); in particular, IGF-1, as we have already seen, among the somatomedins causes an increase in muscle mass (although anabolic properties are attributed to GH, in fact it is IGF-1 responsible for this effect, which, however, is strictly dependent on GH). At the same time, GH, which is not directly involved in protein anabolism, but rather in the ability to increase IGF, together with glucagon, prevents hypoglycemia (they are hyperglycemic hormones) caused by insulin. Absence of carbohydrates that trigger lipolysis. After the introduction of only proteins / amino acids, the plasma glucose concentration cannot be maintained, because glucose is not introduced with the food itself, therefore, hyperglycemic hormones must be secreted primarily> glucagon to stabilize blood glucose levels due to liver glycogenolysis and gluconeogenesis.
Thus, insulin and GH (as well as glucagon) are not always antagonists, but have the effect of Significant synergism after the introduction of proteins only during proteosynthesis and maintenance of glycemic homeostasis. Indeed, only their modern secretion promotes growth, since each of them (actually IGF-1, mediated only by GH) performs a specific activity different from the other, maintaining a different amino acid selection (previously mentioned). Instead, in the absence of protein administration, the action of GH cannot be transformed into protein anabolism, as this action is mediated by insulin and IGF-1. In cases of fasting, when the secretion of GH occurs without synergy of the latter, it plays other metabolic roles, including lipolysis, but not tissue proliferation, as we have already explained.
It is the introduction of carbohydrates that determines the real antagonism between GH (and glucagon) and insulin. Carbohydrates actually strongly stimulate insulin to control blood glucose levels and manage any excess, while GH and glucagon are inhibited since they should not counteract the hypoglycemic action of insulin due to the abundance of glucose, but, on the contrary, the action of insulin does not contrasts, causes a slight accumulation of excess carbohydrates in the form of glycogen and triglycerides. Thus, insulin induces lipogenesis if, in the presence of carbohydrates or carbohydrates, it mixes with other nutrients, while proteins alone do not cause it to store fat, but rather lose weight.
History and clinical use of insulin
Before the discovery of insulin, diabetes was a dangerous disease that could lead to death.
Doctors knew that diabetes was aggravated by sugar, so patients were given strict diets. This method can guarantee the patient a life span of several years, but diet alone has not saved him. Doctors began to realize that diabetics’ pancreas was damaged and malfunctioning, and in 1869 Paul Langerhans, a German, discovered that insulin-producing groups called β-cells are present in the tissue of the pancreas that produces digestive juices. >
The history of insulin is connected with the scientist Nicholas Constantin Paulescu, who was born in Bucharest on October 30, 1869 and died in the same city on July 17, 1931. We are in 1921, and Paulescu, the first in the world, can cure diabetes so much that the next year, or rather April 10, 1922, he received a patent for the discovery of the pancreas. In February 1922, and then more than eight months later, two University of Toronto researchers, Dr. Frederick Grant Bunting and biochemist John James Richard McLeod, published an essay on positive results in normalizing blood glucose in the Journal of Laboratory and Clinical Medicine. obtained in a diabetic dog using an aqueous extract of the pancreas. A long discussion opens up because both researchers seem to have simply put into practice what Paulescu has written in his previous works, and in particular in an essay dated June 22 last year. In fact, both scientists directly refer to this scientific article and claim only to confirm the revolutionary results obtained by Paulescu. In 1923, the Stockholm Nobel Committee awarded the Physiology or Medicine Prize to Banting and MacLeod, completely ignoring Paulescu’s work and research. All controversy and his new works published in the Archives Internationales de Physiologie are useless. Scientist Ion Paul in the seventies under the full Romanian communist regime released a letter dated October 15, 1969, received by Charles H.
Best, an associate of Bunting and MacLeod, in which it is admitted that the two Nobel laureates they did nothing but reproduce Paulescu’s research in laboratories. Be that as it may, the first diabetic patient was successfully treated in 1922; 14-year-old boy who received insulin extract from cow pancreas.
Until the early 1980s, 20 million diabetics worldwide had access only to animal insulin, which is produced by the organs of the bovine and the pig (pancreas). This laborious and time-consuming process resulted in a product that was not ideal for the patient, since in the long term animal insulin is toxic to humans for immunological reasons, causing liver disease and side effects such as blindness, in some cases even death.
With the advent of the biotechnological era, it has become possible to produce insulin by enzymatic modification of insulin produced by pigs or by recombinant DNA technology in bacterial systems, avoiding possible contamination.
Insulin has been produced using recombinant DNA technology since 1982, when the US bacterial system was developed in the US. if. Insulin is associated with the first patent and first marketed biotech drug.
The cloning strategy is to produce chains A and B separately. The information for chain A was synthesized by fusion of the nucleotide sequence from the lacZ gene into the plasmid pBR322, a cloning vector in E. coli. the codon encoding the amino acid methionine was inserted at the melting point between lacZ and the information related to chain A.
Chain B, on the other hand, was synthesized in two steps: first, the N-terminal portion was synthesized using a process similar to that used for chain A; then the terminal C portion was synthesized using the same procedure. After expression of these genes in E. coli , the strand coding fragments were isolated and fused to the lacZ gene by introducing the amino acid methionine at the melting point.
There are many advantages to using the beta-galactosidase system:
- system displayable
- the chains are synthesized by fusion with beta-galactosidase, which has a protective effect against proteolytic degradation.
The two peptides are then treated with cyanogen bromide, a chemical agent capable of cleaving proteolytically cleaved peptides according to the amino acid methionine. All that remains is to purify the synthetics and mix the two chains, allowing disulfide bridges to spontaneously form.
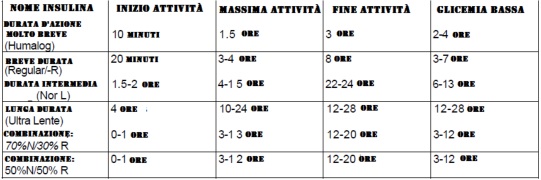
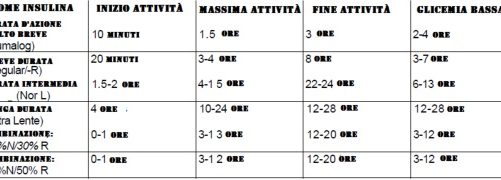
In addition, insulin in solution is in equilibrium between dimeric and esameric forms. In the presence of zinc, it takes the form of an esamer, becoming a more stable but insoluble crystalline or amorphous complex, hence slower absorption. The crystalline form is absorbed more slowly and is known as “ultralent insulin” and its effects are manifested after about 36 hours; the amorphous form is known as “semi-slow insulin”, it is absorbed faster and its action lasts only 12-16 hours; the fast form has a half-life of 4 hours.
However, synthesis using bacteria is very inconvenient for several reasons. First of all, the complexity of assembly (with subsequent low yield) of two chains, since these microorganisms, being prokaryotes, do not possess all the mechanisms of modification and secretion required for a protein, moreover, as a rule, for higher eukaryotes. And then there are big costs for cleaning it. All this can be circumvented by using yeast as bioreactive organisms: being eukaryotes, so they are equipped with a highly developed endoplasmic reticulum and the Golgi apparatus, they will not have problems with the correct assembly and secretion of the protein of interest.
Insulin use in bodybuilding
From the above, it is clear that insulin is the most “non-specific” anabolic hormone (since it is anabolic for both muscle and adipose tissue). The best bodybuilders began introducing insulin into their formulations starting in the 90s along with GH. And if you paid attention to what I previously reported, then the use of insulin by athletes is associated with its ability to increase the intracellular supply of nutrients. Hence, this means that nutrients such as glucose, amino acids and supplements such as creatine and glutamine enter the cell in much greater quantities. Since insulin is not site-specific and that both muscle and fat cells have insulin receptor sites, nutrients are deposited in both these cell types and organs.
The actual subdivision in nutrient storage is highly dependent on receptor sensitivity. For this reason, subjects who develop insulin resistance (receptor insensitivity) tend to gain significant amounts of adipose tissue during insulin administration. Let’s also remember that insulin is both anabolic and anti-catabolic.
Because insulin works synergistically with other anabolic / androgenic chemicals, there are many methods, associations and protocols that different athletes use effectively. There are also minimal “safety” (“safety” is a big word for insulin) protocols that are used by the smartest and best of BodyBuilders:
- A single dose of 1 IU per 7 kg of body weight and no more than twice a day, injected subcutaneously (for a BodyBuilder weighing 90 kg, 13 IU will be used, 90/7 = 13).
- The cycle of use should be 15 to 28 days with a break of 4-8 weeks. Longer periods lose effectiveness (insulin resistance).
- For each IU of insulin injection, at least 10 g of carbohydrates should be consumed (too little supply). Some people say that calories increase too much. I personally disagree and will be left with 15g of carbs per IU of insulin depending on the duration of the insulin used (slow or short).
- The two most effective uses of insulin are the two periods of maximum sensitivity (periods when cortisol levels are also high):
- First dose in the morning (just wake up before eating)
- Immediately after an intense workout.
- AAS improves insulin sensitivity. This means that doses are often lower (less exogenous insulin) when associated with AAS and other anabolic substances.
- Never use insulin protocols in which the half-life of the latter overlaps. When using Humulin-R, which has a half-life of 4 hours, a minimum of 4 hours should elapse between injections.
- Never use insulin without the guidance and supervision of a highly qualified doctor or trainer. Buy and learn how to use a blood glucose test kit.
- Humalog should be prescribed approximately 15 minutes before an adequate meal.
- Regular Type-R was given 30 minutes before an adequate meal.
Improving insulin sensitivity (supplements and medications)
Insulin insensitivity or insulin resistance is a medical situation that is mainly associated with type 2 diabetes. I could talk about the pages of this state, but it is a state in which the insulin receptors of muscle cells become resistant to the circulating insulin released from the intake of nutrients. Because the body has to flush excess blood glucose out of the body, hypersensitive fat cells now contract until they swell and muscle cells barely eat. The situation worsens when hungry and hungry muscle cells continue to experience low glucose levels and liver glycogen stores are depleted. First, the release of muscle protein (catabolism) leads to gluconeogenesis from hard-earned muscle mass. Second, the signal to nourish muscle cells signals the brain to need more food. Third, the fat cells shrink again and the fat cycle starts over.
Many food substrates affect insulin sensitivity in a positive or negative way. Obviously, the goal of all healthy people is to reduce insulin resistance, but the athlete should focus on significantly increasing their insulin. muscle sensitivity to insulin and reduced fat cell resistance. The result is much leaner muscle mass and a significant reduction in body fat.
While there are many additional substrates (see also thiamine and beneficial diabetes supplements) that have some value in this area, some research and experience has shown that they are more effective:
L-Arginine : Taking 2-4 grams of L-Arginine with a protein / carbohydrate drink after exercise is sufficient to increase the rate of glycogen synthesis in healthy people by 30-40%.
L Arginine is also a precursor for the synthesis of nitric oxide (NO). NO plays a key role in every anabolic metabolic pathway in the human body. The addition of L-arginine and NO2 synthesis cofactors (400 mcg folate, 400 mg N-acetylcholine, and 1000 mg phenylalanine) can increase the active life of NO from a few minutes to about 12 hours. Twice a day is the best welcome.
L-Glutamine : This is an amino acid that can be converted to glucose by gluconeogenesis and used for glycogen synthesis. It also acts as a non-insulin dependent mediator to activate cellular glycogen and glucose / amino acid synthesis. This adds to a decrease in insulin resistance. Doses range from 20 to 120 grams per day; 20 grams works well.
Taurine : Taurine is an amino acid that itself is a non-insulin dependent nutrient carrier. This means that taurine can mimic insulin at receptor sites in muscle cells. This increases insulin resistance and helps maintain soluble cholesterol. 2-4g per day with meals is an effective dosage.
chromium picolinate : Improves the sensitivity of the insulin receptor site and to some extent the affinity for the insulin receptor. 200-400 mcg of chromium picolinate per day is an effective dose.
Corosolic acid : Corosolic acid acts in a similar way to insulin, inducing an insulin-like cellular response in muscle cells, but not in fat cells. Reduces blood sugar by about 20% at a dosage of 620 mcg. Corosolic acid is contained in a concentration of 600-620 μg per 50 mg of glucosol powder.
Cinnamon : 1/8 teaspoon of cinnamon 8 times daily with meals can increase insulin sensitivity by up to 300%.
Sage Infusion: (about 60g sage) some studies have shown that it can increase insulin sensitivity by up to 500% (10-20% more likely).
Omega-3 (EPA + DHA) : Increase insulin sensitivity by modulating cellular production of “good” prostaglandins (PG). 2.5 to 6 grams per day of EPA + DHA.
D-pinitol : Of the supplements currently available that are believed to contribute to the sensitivity of insulin receptor sites, D-pinitol has been successfully tested by real world bodybuilders. Although the product is said to mimic the action of insulin, its true value lies in its unique interaction with insulin receptor sites, resulting in a decrease in the amount of insulin required to stimulate the absorption and synthesis of proteins and glycogen. This supplement is classified as Inositol Phosphoglycan. The pharmaceutical equivalent of D-pinitol is called D-chiroinositol. In many studies, D-chiro-inositol has been shown to have a large positive effect on the metabolic rate and absorption of glucose by muscle cells. It has been noted that 50 mg is an effective dose with food.
4-hydroxy-isoleucine : An amino acid found in some foods. The most effective source is an herb called fenugreek. Fenugreek seeds contain 0.8-1.0% 4-hydroxyisoleucine. This means that ground seeds can deliver up to 10 mg per gram. 4-hydroxy-isoleucine influences the transport of glucose and amino acids, having a positive effect on the production of insulin by the beta cells of the pancreas. When 45-90 mg of this substance is taken together with a macronutrient, insulin release can also be increased by 100%, which leads to a significant increase in nutrient transport and absorption in muscle fibers.
Alpha Lipoic Acid. Alpha Lipoic Acid is a compound that plays a key role in the cellular energy metabolism of most living things, from bacteria to humans. Its strong antioxidant properties are also known. For this reason, consuming alpha lipoic acid in the form of a dietary supplement can be very beneficial in activating these vital bodily functions. Alpha Lipoic Acid is not only able to increase the effectiveness of insulin, but it can also improve glucose transport in cells using pathways independent of those of insulin itself. Alpha lipoic acid has the ability to reduce insulin resistance and is excellent at 200 mg daily.
There are also drugs (see also Diabetes: Metformin and Other Drugs) to improve insulin sensitivity:
Glucophage ® (metformin): increases the number and sensitivity of insulin receptor sites; reduces the amount of glucose / sugar absorbed by the intestines; reduces the amount of glucose / sugar produced by the liver (amino acids / proteins are the source for glucose production in the liver). Doses are about 500/850 mg 1-2 times a day.
Avandia ® (rosiglitazone): Increases the number and sensitivity of insulin receptor sites in the cell. Doses are about 2-8 mg 1-2 times a day.
Warning
At the moment it is correct to point out that the use of exogenous insulin and insulin preparations is very dangerous. Misuse of it can lead to coma, brain damage and death. The use of exogenous insulin (or insulin preparations) should only be considered with medical supervision or a qualified instructor! Improper use of insulin can cause diabetes and hyperglycemia.
Other side effects of insulin include:
- sudden sweating
- Heartbeat
- A little excitement
- Mental confusion
- Weakness
- Rage
- Distorted vision
- Difficult to speak.
These side effects indicate that not enough carbohydrates were consumed and not enough often.
Some athletes have found it helpful to keep a vial of glucagon (insulin antagonist) handy in case of any of the above symptoms.
Since exogenous administration of insulin induces suppression of endogenous secretion of this hormone, many athletes, at the end of their insulin cycle, undertake a “pancreatic regeneration” phase with drugs that can stimulate pancreatic secretion. Insulin. The most commonly used is glipizide, a molecule belonging to the sulfonylurea family that has hypoglycemic action and is used as a drug for the treatment of diabetes. It is considered a second generation sulfonylurea, similar to glibenclamide, but with a shorter duration of action.
The hypoglycemic effect is due precisely to the fact that glipizide promotes the release of insulin from the β-cells of the pancreas. In Italy, the drug is marketed by the pharmaceutical company Pfizer under the trade name Minidiab in the form of divisible tablets containing 5 mg of the active ingredient. Its use can lead to side effects such as dyspepsia, nausea, vomiting, gastralgia, abdominal pain, diarrhea, or constipation. Cases of hepatotoxicity with elevated levels of transaminases (AST, ALT, and alkaline phosphatase), acute hepatitis, and cholestatic jaundice are less common. Obviously, if the carbohydrate intake is insufficient, hypoglycemia is a typical consequence of glipizide use.
Conclusion
We talked about the role, function and interaction of growth hormone, insulin-like growth factor 1 (IGF-1) and insulin for tissue growth. We learned that there is a profound synergy between these three peptides and other chemicals. We talked about the history of these hormones, their clinical applications and performance improvements. We also talked about their possible side effects and the short-term dangers of insulin misuse. Hoping that what has been said will help the athlete objectively, and not emotionally reason in the constant search for the “elixir” of growth. There is also hope that prospective athletes or aspiring athletes with natural growth reserves are exploited by 40-50% (or less), with a year of gym experience (or less) and with the belief that chemistry is the solution to everything and that there is no “time spend ”, they understand that progress in bodybuilding goes hand in hand with maintaining optimal health and deep knowledge of training and nutrition.